Dyson, everyone’s favourite vacuum, air purifier and hair dryer brand, has joined the fight against COVID-19. It has refocused its resources to start production of ventilators, an essential piece of equipment in assisting the infected with breathing difficulties. It is also a piece of equipment that is seeing a worldwide shortage as patient numbers grow exponentially.

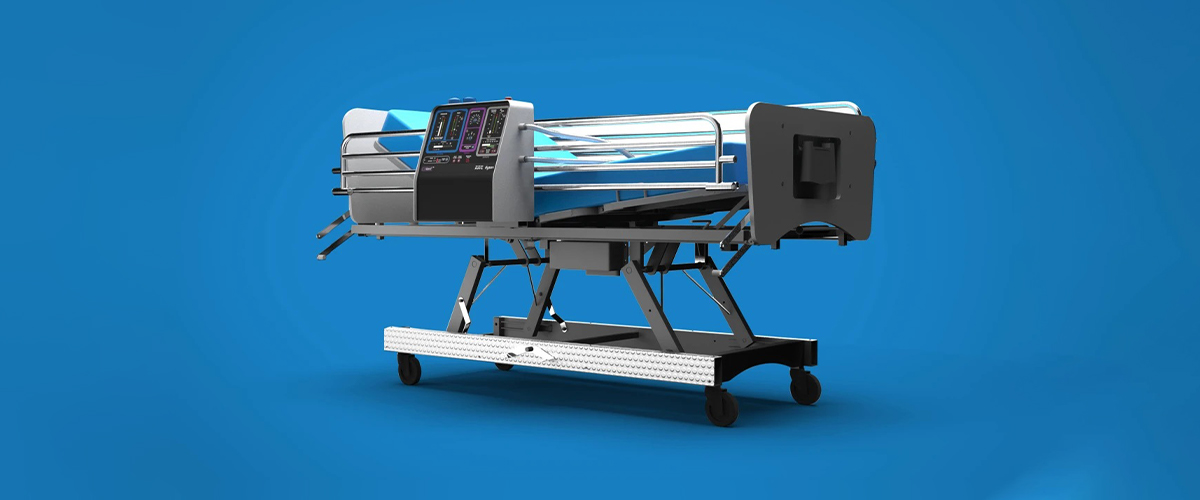
Collaborating with The Technology Partnership in the UK, Dyson has designed and engineered a brand new portable ventilator in a short span of 10 days. Aptly named the CoVent, it is a bed-mounted ventilator that can run on battery power when in field-hospital conditions.
As ventilators are a regulated product, Dyson will be working closely with the UK government and the Medicines and Healthcare products Regulatory Agency to quickly produce a total of 15,000 ventilators; 5000 of which are a donation on Dyson’s behalf. 4000 of these ventilators will be delivered to countries outside of the UK.
Production of the CoVent is expected to begin immediately, although there has not been any word of a specific delivery date. In the current climate, they would want to hurry on up lest the war ends before Dyson is able to deliver.
Below is an internal email sent by founder James Dyson to the company today.
Hospitals are the frontline in the war against Covid-19, where heroic doctors, nurses, and care workers are battling to save lives and help people recover from this terrible virus. As with any battle, there are many challenges to overcome, not least the availability of essential equipment which in this case means ventilators. A ventilator supports a patient who is no longer able to maintain their own airways but sadly there is currently a significant shortage, both in the UK and other countries around the world.
Since I received a call from Boris Johnson ten days ago, we have refocused resources at Dyson, and worked with TTP, The Technology Partnership, to design and build an entirely new ventilator, The CoVent. This new device can be manufactured quickly, efficiently and at volume. It is designed to address the specific clinical needs of Covid-19 patients, and it is suited to a variety of clinical settings. The core challenge was how to design and deliver a new, sophisticated medical product in volume and in an extremely short space of time. The race is now on to get it into production.
The Dyson Digital motor sits at the heart of the new device and the motor’s design is optimised to have a very high level of intrinsic safety, making it particularly well-suited for industrial, high volume production. The device is designed to achieve a high quality air supply to ensure its safety and effectiveness, drawing on our air purifier expertise which delivers high-quality filtration in high-volume products.
Ventilators are a regulated product so Dyson and TTP will be working with the MHRA and the Government to ensure that the product and the manufacturing process is approved. We have received an initial order of 10,000 units from the UK Government which we will supply on an open-book basis. We are also looking at ways of making it available internationally.
I am proud of what Dyson engineers and our partners at TTP have achieved. I am eager to see this new device in production and in hospitals as soon as possible. This is clearly a time of grave international crisis, I will therefore donate 5,000 units to the international effort, 1,000 of which will go to the United Kingdom.
We will keep you updated with our progress.
Best wishes,
James